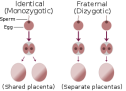Title of study
A randomized, double-blind, parallel-Group Multicenter comparative flexible-Dose Study of Pregabalin Versus Levetiracetam as Adjunctive therapy to reduce seizure frequency in subjects with partial seizures.
Study Number: A0081157
Hospital or Institution
Subject Name
1. Nature and purpose of study
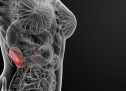
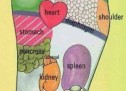
This form is called informative consent form. It contains analytical information for the study in which you’re called to take part, and a consent form which you’ll be asked to sign if you decide to participate. You’re called to take part in a research drug study that is held by Pfizer, because you experiencing partial seizures. Separately from this study you have discussed all available choices with your doctor and have decided to examine the possibility of participation in a clinical test evaluation of two drug effects (of Pregabalin and of Levetiracetam) for your illness. Epilepsy results from abnormal nerve discharges that affect your nervous system. Epilepsy is also known as a problem with seizures. Also, it is localized when a person first arise two seizures at least that were not caused by some known reasons. Most of seizures can be caused by unknown reasons. Seizure is a sudden abnormal explozision electrical activity in the brain that always changes the way by which a person feels or acts in a short period of time. Partial seizures can not be seen easly, while others lead to complete inability. Partial seizures start with electrical discharges in a restricted area of brain. These seizures can be separated further into complex partial seizures and simple partial seizures that are based on loss of vigilance or consciousness. When a seizure is layed out in all tha brain then it is called “generalised”. During the trial it is possible that some or all of these seizures will occur. Pregabalin is approved as an adjunctive therapy in more than 50 countries for therapy of a peripheral nervous pain, as an adjunctive therapy for partial seizure occurrences in adult subjects, and inside of the European Union for therapy of generalized anxiety disorder. Levetiracetam is approved as an adjunctive therapy in many countries for the therapy of partial seizures occurrence in persons aged over 4 and for myoclonic seizures impatients aged over 12 with epilepsy. Aim of this study is to be seen if the effects of a drug (Pregabalin) in comparision with an other drug (Levetiracetam) will help in the control of seizures activity. The study will evaluate also the appearance of any sideffect and whether it is related with Pregabalin and Levetiracetam. The study will also evaluate whether subjects will present improvement in sleep, in general state of health, and in stress and depression. In study, you’ll take either active pregabalin and placebo nor active levetiracetam and placebo. Placebo is an inactive pill that does not contain any drug. You have 50% possibilitiesto get active pregabalin or active levetiracetam. You or your doctor will not know what drug you take. In the case of need your doctor can take directly such kind of information./AlbStroka.com/
© AlbStroka.com
Related posts
-
PËRFITIMET E METODËS ABA NË EDUKIMIN E FËMIJËVE ME NEVOJA TË VEÇANTA
-
RËNDËSIA E VËZHGIMIT TE FËMIJËT
-
MJEKJA QË U MASKUA SI BURRË PËR TË USHTRUAR PROFESIONIN E GJINEKOLOGES
-
NJË NË GJASHTË MESHKUJ DEKLARON SE ËSHTË PËRDHUNUAR NGA NJË FEMËR!
-
SI TË NGADALËSOJMË SHENJAT E PLAKJES
-
GJËRA QË DUHET TË DINI PARA SE TË PËRDORNI NJË MANIKYR
-
JA PSE NUK DUHEN KONSUMUAR VEZËT TË PAGATUARA
-
SASIA E VITAMINËS D PËRCAKTON TREGUESIN E INTELIGJENCËS SË FËMIJËVE!
-
NËSE KENI KËTË GRUP GJAKU MUND TË BËHENI GJITHËDHURUES OSE GJITHËMARRËS
-
JA ÇFARË I NDODH TRUPIT TONË NËSE NDËRPRESIM KONSUMIN E KAFESË!
-
E RRALLË! LINDË FOSHNJË ME DY GOJË!
-
SA LLOJE ORGAZMASH KA?
-
SHKENCA KA PËRGJIGJEN: KJO ËSHTË KOHËZGJATJA IDEALE E NJË MARRËDHËNIEJE SEKSUALE!
-
USHQIMET QË DUHEN LARË GJITHMONË OSE ASNJËHERË
-
KËSHTU PRETENDONIN TË “KURONIN” USHTARËT NGA HOMOSEKSUALITETI!
-
STUDIM I FUNDIT: ÇFARË TREGON MOSHA PËR SHPESHTËSINË E MARRËDHËNIEVE INTIME?
-
PANDEMIA E ETHEVE TË VERDHA
-
SA ZGJATI EPIDEMIA E POLIOMIELITIT?
-
PANDEMIA E VITIT 1918 QË VRAU MBI 20 MILIONË NJERËZ!
-
ALERGJIA E SYVE, SHKAQET DHE TRAJTIMI
-
TEKNIKA EFIKASE PËR ULJEN E STRESIT TË PËRDITSHËM
-
SI TA KUPTONI NËSE FËMIJA JUAJ PO VUAN NGA DEPRESIONI
-
JA SA VEZË MUND TË KONSUMONI NË DITË PA RREZIKUAR TË SËMURENI!
-
SI TË QËNDROJMË LARG NEGATIVITETIT KRONIK
-
BIMA QË SHËRON PROBLEMET E SHIKIMIT
-
PERIMJA QË SHËRON PUÇËRRZAT E GOJËS (AFTET)
-
ÇFARË THOTË SHKENCA PËR BURRAT DHE DASHURINË ME SHIKIM TË PARË?
-
ZBULOHET LIBRI MË I VJETËR MJEKËSOR NË BOTË, DATON 1.600 VJET P.E.S
-
ZBARDHJA E ZONËS INTIME, NDËRHYRJA MË E KËRKUAR PËR MOMENTIN!
-
ÇFARË SHIKOJNË FOSHNJAT GJATË MUAJVE TË PARË TË JETËS SË TYRE?
-
KJO ËSHTË ARSYEJA PSE ERËZA NË FJALË KUSHTON MË SHUMË SE ARGJENDI!
-
SPECIALISTËT PËRGJIGJEN: JA SA FYTYRA MBAN MEND NJERIU MESATAR!
-
DHUNA DHE FËMIJËT!
-
LLOJET E KANCERIT QË PREKIN MË SHUMË GRATË
-
JA CILI VEND MBAN VENDIN E PARË PËR OPERACIONE PLASTIKE!
-
SEKRETI MJEKËSOR I ÇOKOLLATËS SË ZEZË
-
ÇFARË ËSHTË ÇAJI I TULLAVE?
-
CILAT USHQIME PËRMBAJNË SUBSTANCËN QË FAVORIZON ZHVILLIMIN E KANCERIT?
-
SHKENCËTARËT “ZMADHUAN” ALFABETIN E ADN-së
-
A MUNDET NJË PERSON SEROPOZITIV TË BËJË FËMIJË?
-
TË DHËNA TË DOBISHME PËR VODKËN
-
SI TË MBROHENI NGA KANCERI, EKSPERTËT KËSHILLOJNË
-
STUDIM/ PERSONAT ME KËTË TIPAR TRUPOR, RREZIKOJNË TË JETOJNË MË PAK
-
TESTI QË TREGON NËSE VUANI NGA GJËNDRAT TIROIDE
-
STUDIM: SA MË TË GJATË E KENI, AQ MË TË MBROJTUR JENI
-
ZBULOHET VAJI QË LUFTON TULLACLLËKUN NË PAK JAVË
-
AQ SA NUK DINI PËR GJËNDRAT E DJERSËS
-
TË DHËNA INTERESANTE PËR TRURIN!
-
DOBITË E PANJOHURA TË PERIMEVE TË EGRA
-
STUDIM: ZBULOHET BIMA QË ELIMINON KANCERIN BRENDA PAK ORËVE
-
ÇFARË ËSHTË KAFJA E PANGJYRË, KU SHËRBEHET DHE SA KUSHTON?
-
STUDIM: NDJESIA E URISË JU BËN MË TË…
-
E KONSUMOJMË PËRDITË PA E DITUR QË SHKAKTON DËMTIME TRURI DHE SULM NË ZEMËR
-
JA SA KALORI HUMBIM KUR BËN SHUMË FTOHTË!
-
SHKENCËTARËT ZBULOJNË NJË ORGAN TË RI NË TRUPIN E NJERIUT!
-
CILA SUBSTANCË E TRUPIT TONË ËSHTË MË E FUQISHME SE MORFINA?
-
PSE DUHET TË KONSUMOJMË MË PAK VERË TË BARDHË?
-
USHQIMET QË ELIMINOJNË POLIPET E KOLONIT
-
STUDIM: KJO ËSHTË SUBSTANCA QË LUFTON HUMBJEN E KUJTESËS
-
DJATHI – ILAÇI I RI KUNDËR KANCERIT?
-
SA ORË DUHET TË FLENË MESHKUJT DHE PSE?
-
NJIHUNI ME PËRFITIMET SHËNDETËSORE TË HUDHRËS SË ZEZË
-
ZILIA, ELEMENTI MË DOMINANT I PERSONALITETIT TË NJERIUT
-
LLOJET E HORMONEVE FEMËRORE DHE FUNKSIONI THEMELOR I TYRE, (I)
-
LËNGU I KUQ QË DEL NGA MISHI, NUK ËSHTË GJAK!
-
SI TË KURONI DIABETIN NË KUSHTE SHTËPIE DHE PA SHPENZIME
-
EKSPERTËT: SI TË RRISNI VOLUMIN E SPERMËS SË SHËNDETSHME
-
ZBULIM MADHOR: PAJISJA QË MUNDËSON DALJEN E DHËMBËVE TË RINJ!
-
QUMËSHTI I BUBURRECAVE, SUPER-USHQIMI I SË ARDHMES!
-
METALI SUPER I FORTË QË SJELL REVOLUCION NË IMPLANTET MJEKËSORE
-
STUDIMI QË SJELL DËSHMI PËR LIDHJEN MES KËRPUDHAVE DHE TRURIT
-
STUDIM: CILA VITAMINË NXIT SHTIMIN E PUÇRRAVE?
-
STUDIM: USHQIMET QË DUHET TË SHMANGNI PARA RAPORTEVE INTIME
-
STUDIM: NJERIU VIJON “TË JETOJË” DY DITË PAS VDEKJES SË TIJ?
-
ALARM: CILAT JANË TRI LLOJET E MISHIT QË SHKAKTOJNË KRIJIMIN E KRIMBAVE NË TRU?
-
PSE NUK DUHET TA SHUANI ETJEN GJATË NATËS ME UJË TË NDENJUR?
-
SUPERPILULA QË DO TË NA BËJË TË JETOJMË 120 VJET!
-
CILI ËSHTË NDIKIMI I KAFESË NË SHFAQJEN E KANCERIT TË ZORRËS SË TRASHË?
-
Ç’LIDHJE MUND TË KETË ASPIRINA ME BATERINË E MAKINËS?
-
CILAT USHQIME NXISIN GJATËSINË TRUPORE?
-
KALCIUMI RRIT APO ZVOGËLON RREZIKUN E GURËVE NË VESHKA?
-
CILAT USHQIME NUK DUHEN MBAJTUR NË FRIGORIFER DHE PSE?
-
NJIHUNI ME SHENJAT E SHËNDETIT TË KEQ QË DUKEN NË FYTYRË
-
ÇFARË ËSHTË SOMATOTROPINA?
-
CILAT JANË HORMONET E PJESËS SË PËRPARME TË HIPOFIZËS?
-
ÇFARË USHQIMESH DUHET TË KONSUMONI PËR NDËRTIMIN DHE RRITJEN E KOLAGJENIT?
-
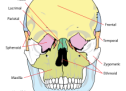
AQ SA DUHET TË DINI PËR KAFKËN
-
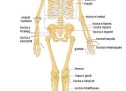
SKELETI I NJERIUT
-
ÇFARË ËSHTË HERNIA DISKALE?
-
KËTO JANË ZAKONET MË TË SHPESHTA QË ÇOJNË NË TKURRJEN E CIPËS SË TRURIT…
-
SHKENCA PËRGJIGJET NËSE MJEKRA E GJATË FAVORIZON APO DËMTON SHËNDETIN E MESHKUJVE
-
STUDIM: MBARTËSIT E KËTIJ GJENI BËJNË VETËM DJEM
-
MJEKIME BIMORE PËR HEMORROIDET
-
KJO ËSHTË BIMA QË ELEMINON KANCERIN BRENDA 40 DITËSH
-
PAJISJA HEMOSTATIKE QË MBYLL PLAGËT BRENDA 20 SEKONDAVE
-
MJEKIME BIMORE PËR ELEMINIMIN E LYTHAVE
-
CILAT USHQIME PËRMBAJNË MË SHUMË HEKUR SESA MISHI?
-
KONSUMI I LARTË I KAFESË FAVORIZON APO ELEMINON RREZIKUN E KANCERIT TË GOJËS?
-
SI TË BËHENI MJEKU I VETES SUAJ
-
SHKENCA DËSHMON SE FEMRAT ME KËMBË TË FORTA…
-
PËRFITIMET SHËNDETËSORE TË KËRPUDHAVE VYSHKJE ZEMRE
-
CILA ERËZË “RIPARON” DËMTIMET E TRURIT?
-
ÇFARË ËSHTË SINDROMA E KASANDRËS?
-
NËSE DONI TË LINDNI DJALË, KY ËSHTË MUAJI MË IDEAL PËR NGJIZJE
-
KJO ËSHTË ERËZA QË NDALON HEMORRAGJINË
-
MJEKIME POPULLORE PËR DHIMBJET E VESHIT, MOSTRETJEN DHE…
-
CILAT KOMBINIME USHQIMESH SHKAKTOJNË ENJTJE BARKU DHE MBAJTJE LËNGJESH?
-
CILËT PERSONA NUK DUHET TË KONSUMOJNË MAJDANOZ DHE PSE?
-
ÇFARË ËSHTË MË MIRË KONSUMI I NJË PORTOKALLI APO MARRJA E NJË TABLETE VITAMINË C?
-
RECETA POPULLORE QË GARANTON HEQJEN E GUNGAVE TË LËKURËS
-
ÇFARË NDODH NË ORGANIZMIN TUAJ SA HERË QË PINI NJË BIRRË?
-
PËRFITIMET SHËNDETËSORE TË TAHINIT
-
JA SI FORMA E NOFULLAVE “DËSHIFRON” GJENDJEN TUAJ SHËNDETËSORE PAS TË 50-ve
-
MAJTAS APO DJATHTAS, SI ËSHTË MË MIRË TË FLINI DHE PSE?
-
CILAT PRODUKTE PËRSHPEJTOJNË PROCESIN E SHËRIMIT TË PLAGËVE/PRERJEVE?
-
VETITË SHËRUESE TË LËNGUT TË DOMATEVE
-
CILI FRUT VEROR ËSHTË CILËSUAR SI “VIAGRA E NATYRËS” DHE PSE?
-
TË HAKMERREM APO TË MOS HAKMERREM?
-
PSE TRISHTIMI ZGJAT MË SHUMË SE ÇDO NDJENJË TJETËR?
-
ÇFARË SËMUNDJESH SHËRON POLENI?
-
DËSHMOHET SHKENCËRISHT SE KANELLA MUND TË PARANDALOJË KANCERIN E…
-
DOBITË QË SJELLË PËRTYPJA E ÇAMÇAKËZIT
-
PËR ÇFARË SHËRBEN KOSI I SKADUAR?
-
LËNGU “MAGJIK” QË UL VLERAT E SHEQERIT NË GJAK
-
JA SI THONJTË E KËMBËVE TREGOJNË NËSE RREZIKONI TË PREKENI NGA KANCERI NË TË ARDHMEN
-
NË Ç’ORË NUK DUHET TË KONSUMOJMË KAFE DHE PSE?
-
NJIHUNI ME PËRFITIMET SHËNDETËSORE TË BIRRËS
-
CILAT SËMUNDJE FSHIHEN PAS ZVERDHJES SË SYVE?
-
SI TË TRAJTONI DHIMBJET MUSKULARE
-
SI TË SHËRONI INFLAMACIONET E GOJËS (MËLLENJA, PLAGË)
-
Ç’SËMUNDJE MUND TË SHKAKTOJË MBIKONSUMI I ÇAJIT JESHIL?
-
DOMETHËNIA E NGJYRËS SË LËNGJEVE VAGINALE
-
MËSONI SE SI PËLLËMBA E DORËS PËRCAKTON GJENDJEN E SHËNDETIT TUAJ! JA TESTI QË DUHET BËRË
-
NË Ç’RASTE DUHET KONSUMUAR UJË I NGROHTË APO I FTOHTË?
-
LËNGU I PORTOKALLIT E RRIT APO E UL TENSIONIN E GJAKUT?
-
SUPER-USHQIMI QË NDËR TË TJERA NGADALËSON DHE PLAKJEN E LËKURËS!
-
SËMUNDJET QË FSHIHEN PAS FRYRJES SË BARKUT
-
CILA PIJE PËRMIRËSON FUNKSIONIN NEUROLOGJIK TË TRURIT DHE KUJTESËN?
-
SHENJAT DHE TRAJTIMI I CERVICITIT
-
DËMTIMET SERIOZE QË SHKAKTOJNË PIJET ENERGJIKE ME APO PA GAZ!
-
CILËT DUHET TË KONSUMOJNË PELTE MBRETËRORE DHE PSE?
-
CILA BARISHTE MEDICINALE RRIT ENERGJINË E TRUPIT NË MAKSIMUM?
-
PËR ÇFARË NDIHMON VENDOSJA E QEPËVE BRENDA ÇORAPEVE?
-
JA ÇFARË TREGON GRUPI I GJAKUT PËR SHËNDETIN TUAJ
-
PËRFITIMET SHËNDETËSORE TË XHENXHEFILIT
-
CILA PJESË E TRUPIT TREGON SE SA GJATË DO TË JETONI?
-
PSE NUK DUHEN KONSUMUAR USHQIMET E RINGROHURA MË SHUMË SE NJË HERË?
-
TRAJTIM I SHPEJTË PËR PICKIMET NGA GRERËZAT
-
KURA POPULLORE PËR TRAJTIMIN E INFEKSIONEVE VAGINALE
-
AQ SA DUHET TË DINI PËR KALIUMIN
-
RECETË NATYRALE PËR HEQJEN E GURËVE NË VESHKA
-
CILAT BIMË ULIN NDJESHËM SHTYPJEN E LARTË TË GJAKUT?
-
PSE DISA PERIME NUK DUHEN KONSUMUAR TË GJALLA?
-
SUPER-USHQIMET QË JU MBROJNË NGA ÇDO SËMUNDJE
-
PSE NA DRIDHEN DUART?
-
SI TË QETËSOHENI NGA DHIMBJET E STOMAKUT
-
ÇFARË JANË LEUKOCITET?
-
VETITË SHËRUESE TË HUDHRAVE
-
KURKUMA, ILAÇI QË I SHËRON TË GJITHA…
-
SA KIKIRIKË DUHET TË KONSUMOJMË NË DITË PËR PARANDALIMIN E VDEKJES SË PARAKOHSHME?
-
KOKTEJI QË SHËRON DHEMBJET E…
-
PËR ÇFARË BËN MIRË TRUMZA?
-
CILI PËRBËRËS I VERËS SË KUQE NDIHMON NË FUNKSIONIN E MIRË TË MËLÇISË DHE HUMBJEN E PESHËS?
-
KONSUMI I PËRDITSHËM I MISHIT TË KUQ MUND TË SHKAKTOJË…
-
CILAT JANË SHENJAT E GODITJES NË TRU?
-
ORARI I GJUMIT SIPAS MOSHAVE
-
PSE NUK DUHET TË KONSUMOJMË SHUMË PRODUKTE BULMETORE?
-
PËRFITIMET SHËNDETËSORE TË KANELLËS
-
CILAT JANË PASOJAT E MBIKONSUMIT TË PESHQVE?
-
PËR ÇFARË BËN MIRË KONSUMI I LËNGUT TË PANXHARIT?
-
ERËZAT QË STIMULOJNË GJENDRAT PËSHTYMORE DHE TRESIN USHQIMET…
-
SUPER-VAKSINA QË ELEMINON TRASHJEN, KUSHTON VETËM 5 EURO
-
MYKU DHE LLOJET E TIJ
-
ÇFARË JANË PROKARIOTET?
-
TONIKU NATYROR QË PARANDALON FTOHJET SEZONALE
-
TË DHËNA ENCIKLOPEDIKE PËR TRUPIN E NJERIUT
-
PIJA QË ELEMINON DHEMBJET E KOKËS
-
BIMËT QË KUROJNË URTHIN
-
SI TË SHKRINI AKULLIN NË NGRIRJE
-
CILI ËSHTË NDRYSHIMI MES KANELLËS ORIGJINALE DHE TË KLONUAR?
-
MËNYRAT QË LARGOJNË AROMËN E KEQE TË GOJËS
-
KURA 1 JAVORE QË ÇHELMON ZORRËN E TRASHË
-
SI TË ÇHELMONI VESHKAT
-
CILA TERAPI E FTOHTË SHËRON DHEMBJET E MUSKUJVE?
-
Ç’lidhje ka nikotina me të ftohtin?
-
SI TË LUFTONI PAGJUMËSINË ME MELATONINË
-
SHURUPI QË DO T’JU SHPËTOJË NGA DHEMBJET E GRYKËS
-
CILAT BIMË NUK KANË NEVOJË PËR UJITJE?
-
MËSONI VLERAT USHQYESE TË RREPKAVE
-
SHURUP NË KUSHTE SHTËPIE PËR TRAJTIMIN E KOLLËS
-
A E DINI SE ÇFARË ËSHTË SËMUNDJA PIKA?
-
ÇFARË KURE DUHET TË NDIQNI PËR KËMBËT E ENJTURA?
-
PËR ÇFARË BËJNË MIRË FARAT E LINIT?
-
SI TË BËNI VICKS NË KUSHTE SHTËPIE, (EKSKLUZIVE)
-
SI TË KENI GJAK TË SHËNDETSHËM
-
SALCA “MAGJIKE” QË PASTRON VENAT
-
CILA PIJE NDIHMON NË ÇHELMIMIN E TRUPIT?
-
NJIHUNI ME VETITË E DOBISHME TË LUIZËS, (EKSKLUZIVE)
-
RECETA QË ZHDUKË XHUNGAT DHE SHENJAT MAVI, (EKSKLUZIVE)
-
ÇFARË TË BËNI QË TË MOS SËMURENI KURRË NGA GRIPI
-
BIMA QË LUFTON EKZEMAT
-
A E DINI SE CILI ËSHTË “SEKRETI” QË FSHIHET PAS RIGONIT?
-
VETITË SHËRUESE TË LAKRËS, (EKSKLUZIVE)
-
AVOKADOJA, “MBUROJA” E MITRËS
-
JA SI FIQTË LUFTOJNË SHTERPËSINË MASHKULLORE
-
“BOMBË” VITAMINASH PËR ORGANIZËM SA MË TË FORTË!
-
CILI ËSHTË MË I DËMSHËM SHEQERI APO KRIPA? SA GR DUHET TË KONSUMOJMË NË DITË?
-
USHQIMET QË “PASTROJNË” MUSHKËRITË NGA TOKSINAT
-
ÇFARË ËSHTË pH?
-
Ç’LIDHJE KANË QEPËT ME QELIZAT?
-
GJËRA QË NUK NJOHIM PËR TRUPIN TONË
-
ÇFARË FSHIHET PAS THARJES SË GOJËS?
-
BOTANISTI 256 VJEÇAR DHE SJELLJET QË SHKAKTOJNË SËMUNDJE…
-
SËMUNDJA E RRALLË QË PO SHUAN NJË FSHAT TË TËRË!
-
BASHKËJETESA E DY GJINIVE NË NJË TRUP, (FOTO)
-
PLUHURI NATYROR QË LUFTON DEPRESIONIN
-
TËRFILI QË SHPËTON JETË, (EKSKLUZIVE)
-
Si sheqeri përshpejton shërimin e plagëve
-
GJITHÇKA RRETH KOLAGJENIT (EKSKLUZIVE)
-
SI TË SHËRONI SHENJAT E PLAGËVE TË VJETRA (EKSKLUZIVE)
-
MËSO BRENDA 5 MINUTAVE NËSE KE AIDS!
-
TERMOMETRI I NDJENJAVE… DASHURIA TË NGROHË VËRTET!
-
HORMONI I MONOGAMISË…
-
QELIZAT E TRURIT JETOJNË MË SHUMË SE TRUPI (JA ÇFARË TREGON NJË HULUMTIM I RI)
-
ERËZAT QË NUK DUHET TË MUNGOJNË NGA ASNJË SHTËPI
-
HUDHRA, MBUROJË E SHËNDETIT TË MIRË
-
ROLI I BAKRIT NË FUNKSIONIN E MIRË TË TRURIT
-
JA SI ERËZAT KUROJNË GODITJET NË TRU
-
AROMA E MOSHËS
-
NË QOFTË SE KËMBËT DO TË KISHIN ZË…
-
KREMI ÇUDIBËRËS QË SHËRON POTHUAJSE GJITHÇKA… (EKSKLUZIVE)
-
SI TË TRAJTONI ANKTHIN, NERVOZIZMIN DHE PAGJUMËSINË
-
ÇAJI I GJELBËR NDËRPRET ZHVILLIMIN E KANCERIT
-
FRUTAT/ZARZAVATET SIPAS MUAJVE TË VITIT
-
Ekziston kujtesa përzgjedhëse, mund të harrojmë ngjarje traumatike?
-
SI FUNKSIONOJNË HEMISFERAT E TRURIT
-
JA SI VAJI I ULLIRIT LUFTON DEPRESIONIN
-
BIMA MREKULLIBËRËSE QË LUFTON AKOMA DHE SHTERPËSINË!
-
CILAT SËMUNDJE QUHEN TË RRALLA DHE SA TË TILLA JANË ZBULUAR DERI MË SOT?
-
NJERIU ME TRE SY (FOTO)
-
Në cilin vend të botës jetojnë më gjatë gratë dhe burrat?
-
Ja si do të jenë gratë në të ardhmen e afërt…
-
JA PSE SPERMA U BËN MIRË GRAVE…
-
Penicillin: the first miracle drug
-
NJERËZ-MAJMUNË JETOJNË MES NESH!
-
NJERIU PA LËKURË!
-
E pabesueshme, është 13 vjeç dhe ngjan 50!
-
E PABESUESHME, KA NJERËZ ME DY BEBËZA SYRI!
-
Binjakët kanë të njëjtin Acid Desoksiribonukleik (ADN)?
-
Cila sëmundje ka vrarë më shumë njerëz?
-
Për ç’arsye kjo bukuroshja nuk del nga shtëpia?
-
U zbulua vaksina kundër kancerit të mushkrive
-
Si të luftoni ASKARIDET (recetë ekskluzive)
-
Κάτοχος του Νόμπελ Ιατρικής 2012: Σε 50 χρόνια από σήμερα οι άνθρωποι θα μπορούν να κλωνοποιούνται…
-
Shpresë e re për të sëmurët me ALZHEIMER
-
NOMA, sëmundja që ha mishin e fëmijëve! FOTO TRONDITËSE!
-
TESTI QË ZBULON KANCERIN E ZORRËS SË TRASHË
-
Studim i ri shkencor ndihmon të verbërit “të lexojnë” Braille!
-
ANALIZË GJAKU TREGON SE KUR DO VDESËSH!
-
Fëmijët e parëlindur kanë tension më të lartë
-
Vetitë terapeutike të ALOE VERA
-
Anomalitë e sindromës Fraser
-
A e dini se çfarë është Sindroma Mobius?
-
FUND MBUSHJEVE TË DHËMBËVE!
-
ÇFARË ËSHTË PRIAPIZMI?
-
Gjithçka mbi vitaminën C
-
SI REDUKTOHET DEGJENERIMI I MUSKUJVE SKELETORË
-
Ginger, një super-ushqim!
-
KAFJA ME QUMËSHT RREZIK PËR SHËNDETIN
-
LEXOJE SE TË INTERESON!
-
SI TË DOBËSOHENI ME JARGË HARDHUCE!
-
DO MBYTESH MOJ E URUAR!
-
Ίσως μπορεί να εμποδιστεί η υποτροπή των τοξικομανών
-
Θεραπευτικά βότανα για διάφορες ασθένειες
-
Mjekët e pandërgjegjshëm dhe pacientët e pafajshëm!
-
ΤΟ ΣΥΝΔΡΟΜΟ ΕΡΓΑΣΙΑΚΗΣ ΕΞΟΥΘΕΝΩΣΗΣ (Burn out)
-
Dita Botërore e Sindromës Down
-
ΥΠΟΦΥΣΗ
-
Ora biologjike dhe sistemi imunitar
-
Kanellë për metabolizëm të mirë
-
Gështenjat: një burim proteinash
-
Pija magjike e Harry Potter-it
-
Vetitë e dobishme të patates
-
Pro apo kundër filmave porno?
-
Luftuesit e kolesterolit
-
Akt barbar rrethprerja e klitorisit
-
Leximi i mendimit!
-
Dobitë e vitheve të bëshme!
-
Sëmundjet infektive pas të 45-ve
-
Konsumi i luleshtrydheve redukton nivelet e kolisterolit në gjak
-
Hipoteroza favorizon jetëgjatësinë!
-
Historia e AIDS-it
-
Uthulla dhe kanceri i qafës së mitrës
-
Forca trajtuese e bimëve
-
Mjalti kundër anemisë!
-
Çfarë është oligomenorrhea/amenorrhea?
-
Panxhari një zarzavat “mitik” me veti të dobishme…
-
Si të bëni scrub me produkte që keni në shtëpinë tuaj…
-
Teknika jo-kirurgjikale per buzë joshëse
-
Seksi mund të na vrasë!
-
Shani se ju bën mirë!
-
Dobitë e puthjes!
-
PSE DISA NJERËZ LINDIN ME GJAK BLU?
-
Uji, sekreti i shëndetit të mirë!
-
Zeolite
-
Quinoa! “Nëna e Drithërave”
-
Gjithçka rreth Anafilaksisë
-
U zbulua ilaçi kundër AIDS-it?
-
Dobitë e shalqiut
-
Karta që diagnostikon HIV-in
-
Transplanti i parë i mitrës tashmë është fakt
-
Dielli redukton rrezikun e shfaqjes së kancerit të gjirit
-
Gjumi i dobëson fëmijët
-
Aspirina lufton edhe osteoporozën
-
SEKS PA TABU PËR ATEISTËT
-
Ushqimet që luftojnë depresionin
-
Qumështorja kuron kancerin e lëkurës
-
Vaksinë e re kundër tuberkulozit
-
Çfarë është Histeroskopia?
-
Kafja kundër diabetit
-
Zbulim i rëndësishëm për artritin
-
Estrogjenet përgjegjësohen për kancerin që prek gratë e reja
-
7 “sekretet” e jetëgjatësisë
-
Trombocite nga staminalet
-
Proteinat e salamandrave eleminojnë kancerin
-
Si të diagnostikoni meningjitin
-
Pini sa më shumë ujë!
-
Zbuluan një gjen të ri që përgjegjësohet për kancerin
-
Mungesa e vitaminave rëndon funksionin e mushkrive
-
Terapia hormonale lidhet me kancerin ovarian
-
Fruktoza ushqen kancerin
-
Astma lidhet me kancerin e mushkrive
-
Orizi i zi, ushqim i mrekullueshëm
-
Krijuan gjak nga lëkura e njeriut
-
Konsumi i kafesë dhe çajit parandalon zhvillimin e kancerit të trurit
-
Selinoja lufton humbjen e kujtesës
-
Luftojnë gurët e veshkave
-
Test i ri për diagnostikimin e kancerit të prostatës
-
Ndotja e ajrit mund të shkaktojë kancerin e gjirit?
-
Partneri ideal
-
Zbulim i rëndësishëm për trajtimin e AIDS-it dhe kancerit
-
“Eliksir jete” me aminoacide
-
Përdorimet terapeutike të toksinës botulinum
-
Kriogjenetika: Sekreti i përjetësisë
-
Konsumoni sa më shumë domate
-
Fruta dhe perime për duhanpirësit
-
Qeliza mëlçie të krijuara nga lëkura e njeriut
-
Kokteile verore
-
HIV/AIDS
-
Ja se ç’duhet të dini për virusin HIV (I)
-
Sa orë duhet të flemë?
-
A e dini çfarë është lecithini?
-
Vaksinë e re kundër kancerit
-
Shpresë e re për ata që vuajnë nga virusi HIV
-
Struktura psikologjike e trurit
-
Mësoni periudhën e menopauzës me një analizë të thjeshtë gjaku
-
Pesha luan rol kryesor në transplantet e veshkave
-
Metodë e re për zbulimin e kancerit
-
Një hap… para jetës artificiale
-
Tre gjenet që fshihen pas dhembjeve të eshtrave
-
U zbulua proteina që lidhet me sëmundjet kardiovaskulare
-
Më pak sheqer në ushqyerjen tuaj
-
U zbulua antidota e kancerit të prostatës?
-
U zbulua ilaçi kundër kancerit të zorrës së trashë?
-
SYN – AKE VEPRON NJËLLOJ SI BOTOX
-
Bima që lufton migrenën
-
Mikrobet në enciklopedi
-
Duhanpirja kundër IQ (treguesit të inteligjencës)
-
Pajisje e re për matjen e fertilitetit
-
Dita Botërore kundër Kancerit
-
Shpresë për personat me dëmtime të trurit
-
I rrezikshëm përdorimi i bimëve për epileptikët